Describe the Energy Needed to Get a Reaction Started
Effects of large families on child development 5. The rate of reaction increases if the activation energy decreases.
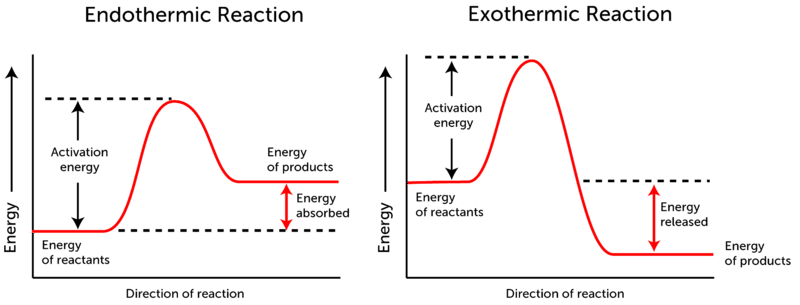
Chemical Reactions And Energy Ck 12 Foundation
Energy must be added to the reactants to overcome.

. Well the activation energy is the extra energy given to get useful work done. In chemistry we call it the minimum amount of energy or threshold energy needed to activate or energize molecules or atoms to undergo a chemical reaction or transformation. The energy needed to get a reaction started is called activation energy.
This question involves a common error in thinking about chemical reactions. Less urbanization and crowding d. What is the term used to describe the energy needed to get a reactions started.
What is the term used to describe the energy needed to get a reaction started a. Activation energy If a reaction in one direction release energy the reaction in the opposite direction. Helpful 0 Not Helpful 0 Add a Comment.
Activation Energy is the minimum amount of energy needed to start a chemical reaction. The carrying capacity of Micos aquarium is for five fishes only but he placed ten fishes instead. Over supply of job opportunities b.
What is the term used to describe the energy needed to get a reaction started. The energy needed to get a reaction started is called activation energy. All chemical reactions need a certain amount of activation energy to get started.
A cohesion energy b chemical energy c activation energy d adhesion energy. This energy which is recovered as the reaction proceeds is called. Describe the effect on the resources if the population of fishes is above the carrying.
Correct answer to the question What is the term used to describe the energy needed to get a reaction started. The energy required to begin a reaction is called activation energy. Answer 1 of 3.
To overcome an energy barrier between reactants and products energy must be provided to get the reaction started. Activation energy is the term used to describe the energy needed to get a reaction started. This certain energy should be reached for the reaction to proceed.
The activation energy units are LCalmo KJmol and Jmol. Usually once a few molecules react the rest will quickly follow. What is the term used to describe the energy needed to get a reaction started.
The first few reactions provide the activation energy for more molecules to react. There is an energy barrier that separates the energy levels of the reactants and products. The rate of reaction is given by the Arrhenius equation.
I think the correct answer from the list of choices above is option B. What is the term used to describe the energy needed to get a reaction started. Increased equalities in education c.
Hydrogen and oxygen can react to form water. The activation energy is the energy required to start a reaction. Save teachers time and engage students with a new simpler interface.
The energy needed to get a reaction started is the A. When a reaction starts activation energy is needed. It is understandable because we know that paper does not burn unless you.
Enzymes are proteins that bind to a molecule or substrate to modify it and lower the energy required to make it react.

Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy

Energy Diagram Overview Parts Expii

What Is Activation Energy Definition And Examples Energy Activities Activities Energy

0 Response to "Describe the Energy Needed to Get a Reaction Started"
Post a Comment